Technology
A breakthrough in the fight against bacteria that cause ‘flesh-eating’ diseases
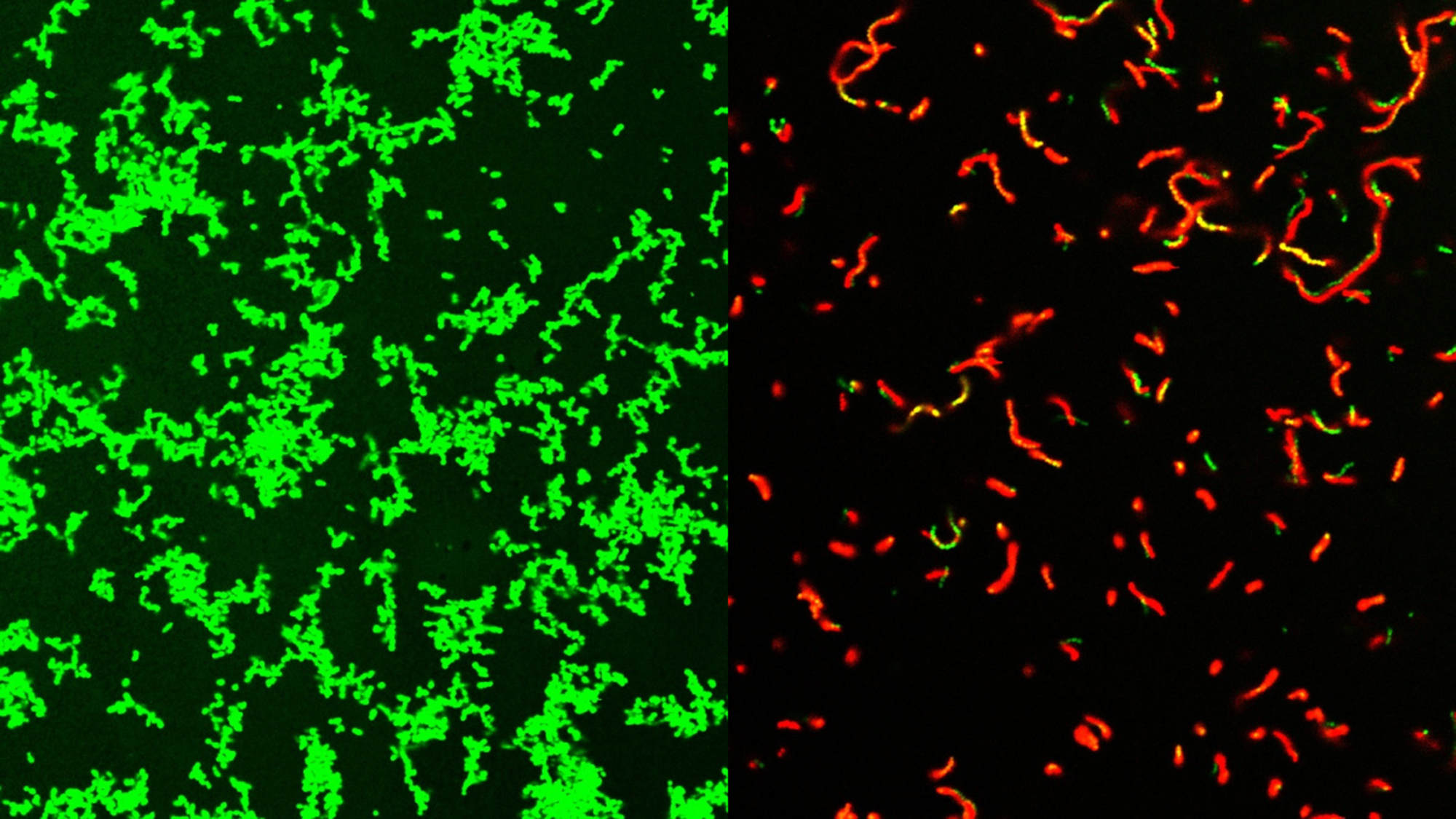
An international team of scientists has developed a new family of compounds that can eliminate bacterial infections in mice. Some of these infections can result in serious ‘flesh-eating’ diseases. There are about 700 to 1,100 cases of flesh-eating diseases per year in the United States. The new family of compounds could also mark the beginning of a new class of antibiotics and is described in a study published Aug. 2 in the journal Scientific progress.
Growing resistance
For decades, doctors have been sounding the alarm about pathogens becoming increasingly resistant to currently available drugs. This makes them more dangerous and, according to the Centers for Disease Control and Prevention (CDC), over 2.8 million antimicrobial resistant infections occur every year in the US. More than 35,000 people die of these infections. To combat this, newer antimicrobials will be needed to replace those to which bacteria have become resistant.
Molecular microbiologists Scott Hultgren and Michael Caparon from Washington University School of Medicine in St. Louis and chemist Fredrik Almqvist from Umeå University in Sweden collaborated on this new family of compounds called GmPcides.
[Related: These flesh-eating bacteria are finding new beaches to call home.]
GmPcides work by being goal-oriented gram-positive bacteria. These types of bacteria can cause various drug-resistant staph infections, toxic shock syndrome, and other bacterial diseases that can be fatal.
“All gram-positive bacteria we tested are sensitive to that substance. That includes enterococci, staphylococci, streptococci, C. difficultwhat are the most important pathogenic bacterial types,” Caparon said in a statementT. “The compounds have broad-spectrum activity against numerous bacteria.”
A ‘happy accident’
The new GmPcide compounds are based on a type of molecule called ring-fused 2-pyridone that was developed by what the team calls a happy accident. Caparon and Hultgren had asked Almqvist to develop a chemical compound that could prevent bacterial films from adhering to the surface of urethral catheters. These are common ones cause of urinary tract infections in hospitals.
The resulting compound also had infection-fighting properties against multiple types of bacteria. Their previous studies showed that GmPcides can kill bacterial strains in Petri dish experiments.
In this new studythey took those petri dish experiments one step further by testing how compounds work in necrotizing soft tissue infections. These rapidly spreading infections usually involve multiple types of gram-positive bacteria. Necrotizing fasciitis–or flesh-eating disease– is the best known of these infections. It can quickly damage tissue so severely that limb amputation is often necessary to control its spread. About 20 percent of patients die with a flesh-eating disease.
The team focused on one pathogen responsible for approximately 500,000 deaths annually:Streptococcus pyogenes. A group of mice were infected with S. pyogenes. One group was treated with GmPcide, the other was not. Those given the GmPcide treatment fared better than the untreated mice in almost every way. They lost less weight, had smaller ulcers and fought the infection faster. Damaged areas of the skin also seemed to heal more quickly after the infection.
Although it is still not entirely clear how GmPcides did all this, microscopic examination showed that the treatment has a significant effect on bacterial cell membranes. These are the outer coverings of the microbes.
[Related: ‘Bacterial glitter’ shimmers without pigments.]
“One of the tasks of a membrane is to keep outside material out,” says Caparon. “We know that within five to 10 minutes of treatment with GmPcide, the membranes become permeable and allow things that would normally be excluded to enter the bacteria, indicating that those membranes are damaged.”
This can alter the bacteria’s own functions, including actions that damage the host and make the bacteria less effective at undermining the host’s immune response to infections.
GmPcides are also less likely to lead to drug-resistant strains. The experiments designed to create resistant bacteria showed that very few cells can resist the treatment. This means they are less likely to pass on their benefits to the next generation of bacteria.
The road ahead
According to Caparon, there are still plenty of steps before GmPcides is available at your local pharmacy. The team has patented the compound used and licensed it to QureTech Bio, a company that does just that Caparon, Hultgren and Almqvist have an ownership interest in it. The license was conditional on the expectation that they would partner with a separate company that could manage pharmaceutical development and clinical trials to bring it to market.
According to the team, the kind of collaborative science that GmPcides has created will be needed to address problems such as antimicrobial resistance.
“Bacterial infections of all types are a major health problem, and they are becoming increasingly resistant to multiple drugs and therefore more difficult to treat,” Hultgren said in a statement. “Interdisciplinary science facilitates the integration of different fields, which can lead to synergistic new ideas that have the potential to help patients.”













