Health
Dangers of people using animal medicines and vice versa
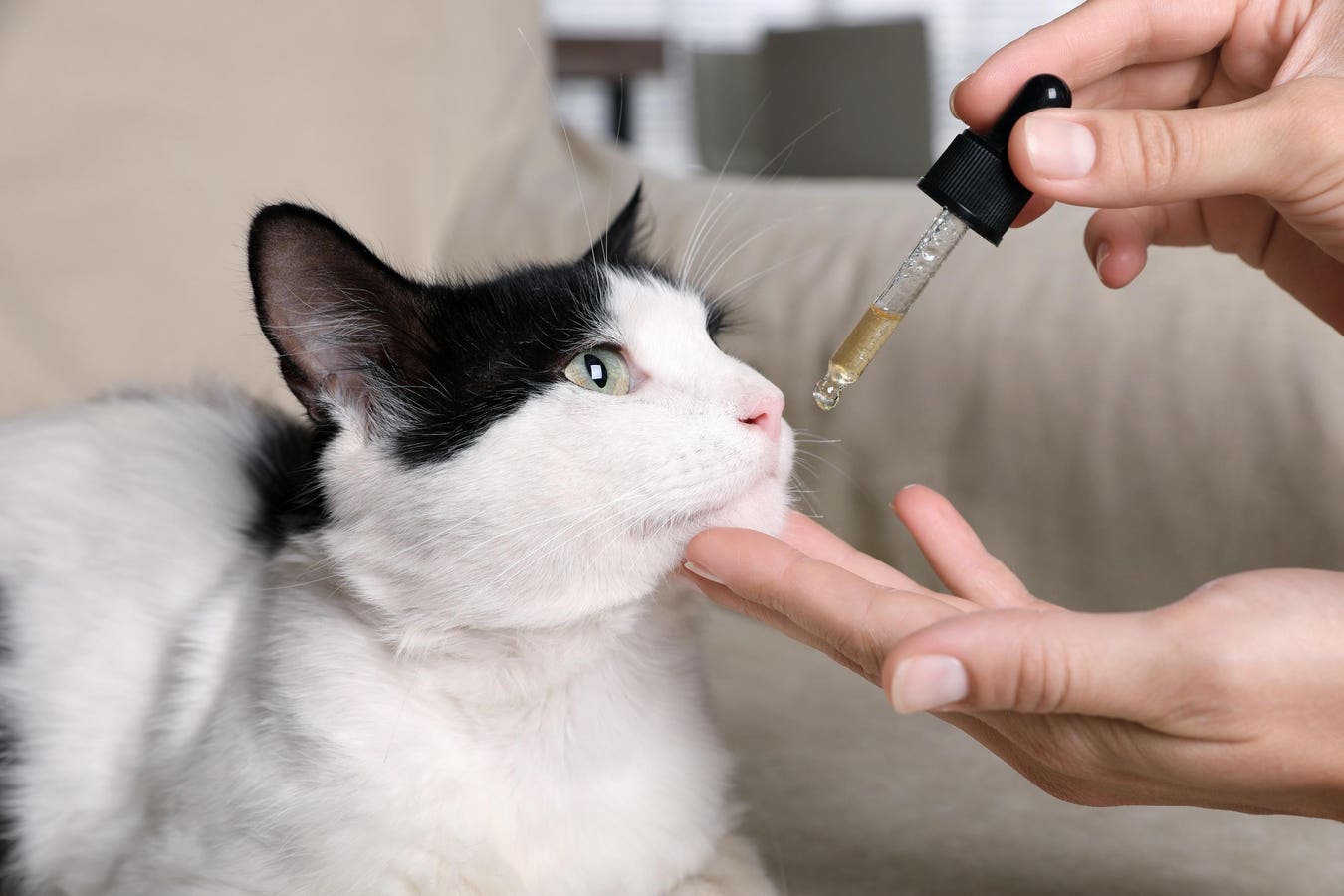
A woman gives a tincture to a cat.
Prescription drugs intended for use in veterinary medicine can be dangerous to humans. Conversely, medications approved for human use can pose a serious danger to animals. And at the same time, some drugs can be prescribed to humans and animals and work for both, as long as it is done with the correct dosage and formulation.
Have you ever wondered if your cat might be taking the same medications as you? For example, cats can be administered transdermal doses of mirtazapine (brand name is Remeron or Mirataz) as an appetite stimulant. Mirtazapine is approved by the Food and Drug Administration for the treatment of depression in humans. Appetite stimulation and weight gain are common side effects and therefore it may be beneficial for patients experiencing weight loss and loss of appetite. Similar benefits can also be achieved for animals.
Veterinarians can legally prescribe an approved human drug, such as mirtazapine, to animals under certain circumstances. This is called an extra-label use by the American Veterinary Medical Association explains. It involves prescribing an approved drug in a manner that deviates from the drug’s approved labeling, yet still meets the conditions set by the Animal Medicinal Drug Use Clarification Act of 1994 and FDA regulations. Deviations from FDA-approved labeling here include use in a different species, for a different indication, at a different dose, frequency, or route of administration.
The FDA is the legal authority in the US to approve and regulate medications for both humans and animals. A medicine intended for use in animals is called a new veterinary medicinal product. The FDA has one division called the Center for Veterinary Medicine that approves and regulates new veterinary drugs. And because each species responds differently to drugs due to differences in physiology and metabolism, the FDA determines whether a drug is safe and effective for a specific use in a particular species.
According to the National Community Pharmacists Association, the following four drugs developed and approved for humans are common administered to certain animals under the extra-label section: Diphenhydramine to help treat allergies, allergic reactions and motion sickness; hydrocortisone for raw, itchy, or irritated skin; famotidine as a stomach acid reducer; and dimenhydrinate for motion sickness, although a better choice might be an FDA-approved animal treatment such as Cerenia (maropitant citrate).
But you never want to play vet with the human medications in your medicine cabinet. Consult your veterinarian before using any of the above medicines on your pets.
Also, you should not take medications intended for animal use just because you may recognize a known active ingredient. In other words, people should not use products marketed for veterinary use that have not been evaluated by the FDA for human safety or are otherwise unsuitable for human consumption. These products can cause adverse effects, including serious illness and death, when ingested by humans. Either the veterinary medicinal product itself poses a significant risk to humans, or the dosage or formulation.
Take xylazine for example. It is a non-opioid veterinary tranquilizer approved for use in animals, but not humans. Not only is the animal tranquilizer dangerous when ingested by itself by humans, fentanyl mixed with xylazine is also known on the street as “tranq” and is causing an alarming increase in overdoses and deaths.
Another example is the dissociative, hypnotic drug ketamine, which can be prescribed to people for certain mental disorders. But ketamine has also become an illegal street drug used illegally for recreational purposes. There are veterinary and human formulations of ketamine. The veterinary formulations are ten times stronger, making it potentially fatal if ingested by humans.
The reverse is also true: when animals consume products intended solely for human use, they can suffer harmful consequences, such as serious illness and death. Some medications are highly toxic and potentially fatal to cats, for example bismuth subsalicylate and acetaminophen. The FDA lists medicines for which extra-label use in animals is prohibited.
In other cases, a drug such as fluoxetine – the active ingredient in the widely used antidepressant Prozac – may be approved for label use in both humans and animals. For “lonely dogs with separation anxiety,” Eli Lilly launched Reconcile in 2007, which contains fluoxetine and is specially formulated for animals. Dogs are often prescribed fluoxetine. This is not a new phenomenon. Fifteen years ago the New York Times published one article about the increase in the prescription of medicines intended for humans to animals.
And then there is the special case of the broad-spectrum antiparasitic ivermectin, which has long-approved uses in humans and animals. However, animal-based ivermectin products differ greatly from those approved for humans. In veterinary medicine it is indicated for prevention and treatment heartworm and intestinal worms. You may have seen advertisements on television for Heartgard, which prevents heartworm disease in dogs and treats and controls intestinal worms in animals. One of the two active ingredients in Heartgard is ivermectin.
In humans, a topical ointment containing ivermectin may be prescribed to treat problems with lice and rosacea, while a tablet is used for parasites including intestinal strongyloidiasis and onchocerciasis.river blindness) And lymphatic filariasis.
During the Covid-19 pandemic, a controversy erupted around the use of ivermectin in humans to treat or prevent Covid-19. The FDA warned Americans in December 2021 not to use ivermectin for this purpose. The FDA stated that there was no evidence to support the use of ivermectin against Covid-19.
The agency continued reminds us “Never use medicines intended for animals, on yourself or on other people.” While the latter statement is valid, it does not technically apply to ivermectin since the product is not just for animals. Furthermore, the FDA’s legal authority does not extend to providing medical advice or discouraging off-label use of medications.
Although the FDA does not approve ivermectin as a treatment for Covid-19, doctors can still prescribe it if they insist. ineffective it appears to be for patients, as has been the case recorded repeatedly separately peer-reviewed studies during recent years.
The FDA threw down the gauntlet when it posted a tweet in 2021 opposing the use of ivermectin: “You are not a horse. You are not a cow. Seriously everyone. Stop it.” This angered some doctors who believed the FDA had overstepped its bounds. In a lawsuit, the FDA has since agreed to remove and never republish the infamous tweet and other similar posts on social media, according to Newsweek.
Broadly speaking, we can say that medications approved for use in both humans and animals can be prescribed, as long as they are in the correct dosage and formulation. Furthermore, human medications can be used in limited cases for pets and other animals – under extra-label regulations – but only if recommended by your veterinarian and dosed and formulated appropriately. Finally, medicines intended solely for use in animals should not be taken by humans.