Health
No link has been found between anti-obesity medications and suicidal thoughts

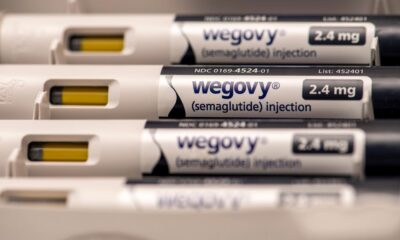
LONDON – After a nine-month investigation, European regulators said Friday they have found no evidence that GLP-1 drugs such as Ozempic and Wegovy cause suicidal thoughts or actions.
The European Medicines Agency’s announcement reflects the findings of a study by the US Food and Drug Administration, who said in January no causal relationship was found between weight loss class and diabetes medication and suicidal ideation.
The EMA’s review began last July after anecdotal reports of patients experiencing thoughts of self-harm while taking liraglutide (a branded version is Saxenda from Novo Nordisk) and semaglutide (Ozempic and Wegovy from Novo). In November, the EMA’s Pharmacovigilance Risk Assessment Committee asked the makers of some GLP-1 medicines for additional details.
As part of their review, the committee analyzed medical records, clinical trials, post-marketing surveillance data and other studies, including a recent Nature Medicine article which showed that GLP-1 drugs carry a lower risk of suicidal ideation than other treatments for obesity and diabetes. Overall, the committee said the results of their review “did not support a causal relationship between the use of GLP-1 receptor agonists and this risk” of suicidal ideation.
The EMA said GLP-1 makers should continue to monitor for any cases of suicidal thoughts or actions in the future.
GLP-1-based drugs such as Ozempic and Wegovy and Eli Lilly’s Mounjaro and Zepbound have exploded in popularity in recent years. However, as more patients began taking the drugs, a number of anecdotal reports of suicidal thoughts emerged, which led to the studies.
In its January statement, which was based on a preliminary study, the FDA said it could not definitively rule out a small risk of suicidal thoughts and actions. It said the investigation was continuing.
Previous obesity treatments were dogged by fears of psychiatric risks. For example, rimonabant was withdrawn from European markets in 2008 over concerns that it increased the risk of suicidal thoughts.
The FDA labels for Wegovy and Zepbound, which are approved for obesity, already warn doctors to monitor patients for suicidal ideation, while the labels for Ozempic and Mounjaro, which are approved for type 2 diabetes, do not include that warning.