Health
Nvidia, Biogen, Ionis, AstraZeneca, ChatGPT
Want to stay up to date on the science and politics driving biotechnology today? Sign up to receive our biotech newsletter in your inbox.
Ask again! Can you tell us what you think of The Readout? Fill this in questionnaire!
Today, some interesting tidbits from STAT’s Breakthrough Summit West in San Francisco, AstraZeneca’s antibody helped prevent Covid-19 in immunocompromised patients, and more.
Google and NVIDIA say AI is already impacting drug discovery
Artificial intelligence is already making its mark on drug discovery, leaders from NVIDIA and Google said yesterday at STAT’s Breakthrough Summit West. We don’t have an AI-developed drug yet. But there have been more studies on new drug applications to the FDA that mention AI, write STAT’s Katherine MacPhail and Mario Aguilar. And some early-stage readouts, aided by AI, may show signs of success.
“We will, hopefully in ten or fifteen years, reach that medicine n-of-1. If the process and production of drugs can really be reduced, we don’t have to worry about the economies of scale of drug production. We can actually build and produce those drugs,” said an NVIDIA executive.
Read more.
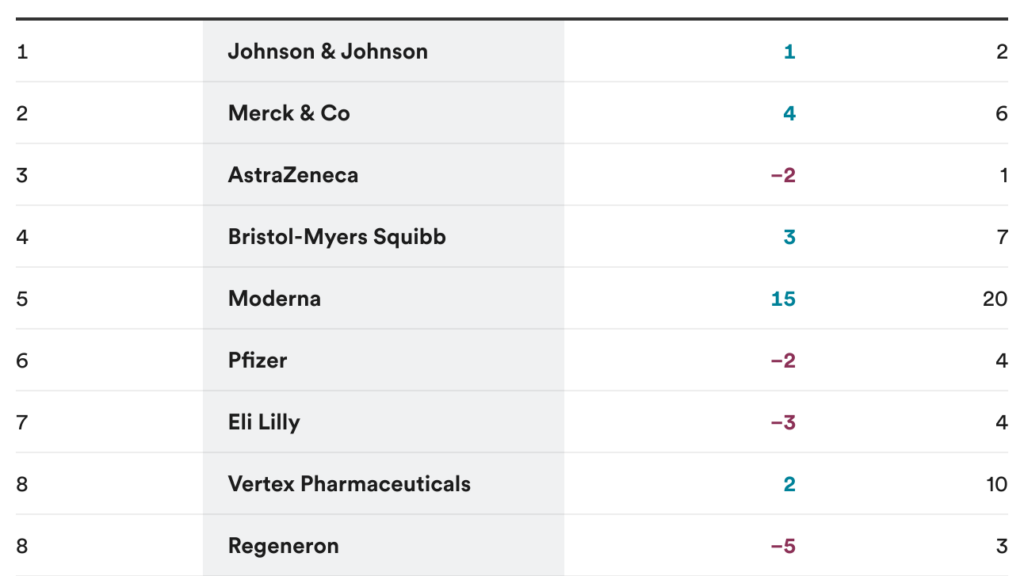
The most innovative and inventive drug manufacturers
Which drug makers are the best R&D performers – and how do you analyze innovation from inventions? The 13th Pharmaceutical Innovation and Invention Index, published at the STAT Breakthrough Summit West, makes this distinction in a ranking of the largest pharmaceutical companies.
The companies labeled as particularly inventive are J&J, Merck and AstraZeneca – because of their luxurious pipelines and new experimental drugs. Meanwhile, Novo Nordisk, GlaxoSmithKline and Pfizer were seen as excellent for innovation over the past year, thanks to their ability to bring new drugs to market.
Read more.
AstraZeneca antibody reduced risk of Covid-19 in immunocompromised people
From STAT’s Jason Mast: AstraZeneca said a new long-acting antibody reduced immunocompromised patients’ risks of contracting symptomatic Covid-19 infections in a large phase 3 prevention trial.
That could mean a new option for vulnerable individuals — such as certain cancer patients and transplant recipients — who have been waiting for drugs that could provide extra protection since Evusheld, AstraZeneca’s previous antibody, was pulled from the market in early 2023 due to new variants. One new option was already approved last month.
Yet it is not clear whether the new antibody, sipavibart, will actually reach the market. SARS-CoV-2 has evolved since the company began developing the drug and sipavibart is not thought to effectively neutralize variants with a mutation called FL456, which now appears in many circulating variants.
The study had two primary endpoints: disease reduction in any variant, and disease reduction in variants without the FL456 mutation. AstraZeneca said sipavibart met both endpoints. It has not yet been said how sipavibart fares against variants with FL456 mutations. Regulators will probably want to know this.
Live! From STAT Breakthrough Summit West
On this week’s ‘Readout LOUD’ we’re live in San Francisco at STAT’s Breakthrough Summit West. AI is a big theme everywhere this year, so on that note we talk to our AI correspondent – and recent Pulitzer finalist – Casey Ross, who took the stage to discuss AI-centric drug discovery with both NVIDIA and the Google-backed Isomorphic Laboratories.
We also discuss this week’s biotech news, including more data on anti-obesity drugs, this time from Roche, and the completion of Novartis’ acquisition of MorphoSys.
Listen.
Biogen, Ionis genetic medicine fails in ALS research
An attempt by Ionis and Biogen to treat non-genetic forms of ALS with an experimental gene-targeting drug has failed. The companies tested a drug, BIIB105, in a 99-patient study, but it showed no improvement in patients – so they stopped further trials.
Only about 10% to 20% of ALS cases are genetic, but promising preclinical research suggests that knocking out the ATXN2 gene could help any patient who develops ALS, writes STAT’s Jason Mast. And while the drug did indeed reduce ATXN2 levels in the spinal fluid, it did not appear to slow the death of patients’ neurons.
Ionis still has a treatment for FUS-driven ALS, a genetic form of the disease, in phase 3 trial. The NIH is funding efforts to create customized drugs for the extremely rare genetic causes of ALS.
Read more.
Read more
- Microsoft’s Peter Lee says ChatGPT should not be used for the initial diagnosis, STAT
- PDS Biotech’s survival advantage is absurdly overstated, STAT
- Amgen drug receives US approval for advanced lung cancer Bloomberg

