Health
The push for universal blood cancer therapy
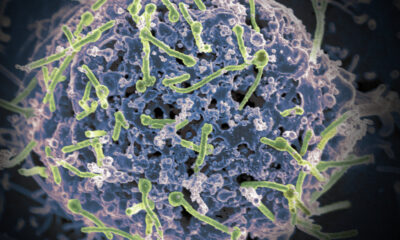
Cancer cell amid flowing red blood cells.
In April 2012, Emily Whitehead, a seven-year-old girl with acute lymphoblastic leukemia, became the first pediatric patient ever to receive an experimental new treatment for blood cancer.
For years, the mainstream approach to treating blood cancer has been to use very high doses of chemotherapy to eradicate the patient’s entire blood cell production system, along with the cancer cells. A bone marrow transplant from a suitable donor is then needed to restore the patient’s ability to produce blood cells. All in all, the process is grueling and comes with many side effects.
In Whitehead’s case, her disease had become resistant to chemotherapy, making her ineligible for a bone marrow transplant. Given only a few weeks to live, doctors decided to try CAR T-cell therapy, a then-new process that collected her T cells, genetically reprogrammed them to recognize her cancer, and modified these T cells were reintroduced into her blood. It worked – Whitehead went into remission and a new era of blood cancer medicine was born.
Now twelve years later they are there six CAR T cell therapies approved by the U.S. Food and Drug Administration, but there is an important limitation: each therapy must be specifically tailored and tested for an individual patient’s disease, a laborious, expensive and time-consuming process. Last year, a study emphasizes that patients wait an average of six months before starting treatment, with a quarter of them dying in the meantime. “Anything we can do to improve cell technology for cancer patients can’t come soon enough,” said Fyodor Urnov, professor of molecular and cell biology at the University of California, Berkeley.
But around the world, from Switzerland to the US, scientists are pursuing a paradigm-changing approach using a next-generation form of gene editing called base editing, with the aim of minimizing the side effects of treatments and ultimately being able to form of blood processing. cancer.
At the end of May, researchers from the University of Basel published a study in nature apply their approach in animal models and human cells. Using a protein target called CD45, which is found on the surface of all blood cells, a targeted form of chemotherapy, delivered via an antibody-drug conjugate (ADC), is used to destroy all diseased cells. At the same time, a stem cell transplant is performed, but these donor stem cells are genetically modified to be invisible to the ADCs, an approach they call shielding.
“The idea is to create a new blood system that is resistant to the treatment we apply,” says Professor Lukas Jeker from the University of Basel, who led the research. “It’s made possible by these newer versions of genome editing, very precise tools that allow for very small, targeted changes. We can edit the stem cells in such a way that the ADC can no longer bind, but the function of the cells is retained.”
Last summer, researchers from the University of Pennsylvania School of Medicine published a similar study in which they used base editing to manipulate T cells to find the CD45 target on blood cancer cells. Once again, a stem cell transplant takes place at the same time, but the stem cells have also been processed at the base to keep them protected from the wandering T cells.
“The shielding is technically a very stylish idea,” says Urnov. “One of the most powerful implications is that you can actually harvest the patient’s own bone marrow cells, edit them to protect them from the therapy, and put them back in.”
Improving patient access
Rachel Haurwitz, president and CEO of Caribou Biosciences, a company developing its own off-the-shelf CAR T-cell therapies to treat blood cancers, described the idea of a universal blood cancer therapy as intriguing and an example of the progress that has been made on the field of genome editing.
“First-generation CRISPR-Cas9 technologies were very good at genome editing for academic or laboratory purposes, but often led to genome editing on unintended targets,” she says. “Advances in CRISPR have dramatically reduced these errors. [However] I believe that a combined CRISPR-edited CAR T cell and hematopoietic stem cell (HSC) approach would require significantly more assessment in animal or non-clinical models, especially by examining the long-term effects of transplanting edited HSCs, in the hope of reducing risks to patient safety.”
Jeker has founded a spin-off company called Cimeio Therapeutics with the aim of pushing the approach into the clinic while continuing to conduct more preclinical studies in his research lab.
In addition to cancer, he believes the same approach could be used for a number of autoimmune diseases such as lupus, which is caused by lymphocytes reacting to the body. “We could use this approach to actively deplete autoreactive B cells and T cells with a drug therapy, while replacing them with a new immune system adapted to protect it from the drug,” he says. “So it will be a very deep reset of the immune system. Multiple sclerosis is another autoimmune disease with good mouse models in which we could test the approach.”
For blood cancer patients, Haurwitz says the goal of universal treatment should be an off-the-shelf therapy that can be easily made available to all patients at any time, something Jeker says should be possible with his approach since ADCs by definition , ready-made treatments, while genetically engineering stem cells only takes a matter of days. Haurwitz points out that the problem with many current CAR T-cell therapies is not only the lengthy manufacturing process, but also the fact that delivering a customized patient-specific cell therapy product is only possible at large, high-end healthcare facilities.
“If a universal CAR T cell is created for all blood cancers, that product should be readily available to patients regardless of their position on the production line, if they are close to a center of excellence, or if they a cancer that progresses quickly,” she says. “Developing a CAR T-cell therapy that can treat any blood cancer would be incredible, but only impactful if patients can receive that therapy.”
Meanwhile, Emily Whitehead just completed her freshman year at the University of Pennsylvania, near the medical center where she was treated with CAR T-cell therapy as a child. Her remarkable story is proof that scientific breakthroughs can save lives and change medicine forever.
Thanks to David Cox for additional research and reporting on this article.