Health
Lilly, Bristol Meyers Squibb, Johnson & Johnson

Want to stay up to date on the science and politics driving biotechnology today? Sign up to receive our biotech newsletter in your inbox.
Hi, I got up bright and early today to report on the earnings, with the help of some coffee (with pasteurized milk) and the soundtrack for the new Challengers movie.
And if you haven’t already, fill it out this survey so we can make this newsletter even more useful for you!
Lilly raises expectations for 2024, even if supply is limited
Here are the key results from Lilly’s earnings this morning:
- Adjusted 2024 earnings estimates raised to a range of $13.50 to $14.00 per share, compared to a previous forecast of $12.20 to $12.70 per share
- First-quarter earnings were $2.58 per share, beating expectations of $2.39 per share
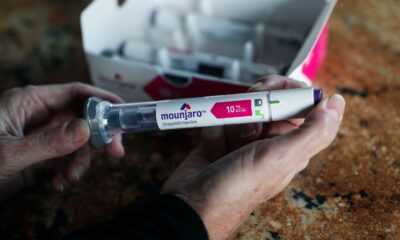
- Obesity drug Zepbound brought in $517 million in revenue in the first quarter, more than the $389 million analysts expected.
- But sales of sister diabetes drug Mounjaro missed: It posted revenues of $1.8 billion, lower than the $2.1 billion analysts had forecast.
Lilly, as well as its competitor Novo Nordisk, are hampered by supply constraints. They are both rapidly scaling up production capacity, and Lilly said it expects the biggest production increases this year to come in the second half.
Read more from me about the earnings results and how Lilly is responding to the supply challenges. And come back after the earnings call starting at 10am ET.
Judge slams drug makers and challenges IRA
A federal judge ruled yesterday against a complaint filed by Bristol Myers Squibb and J&J aimed at dismantling Medicare’s new drug price negotiation authority created by the Inflation Reduction Act.
The pharmaceutical giants argued that the bargaining program is an unconstitutional government seizure of their drugs, a violation of their right to freedom of speech and an unconstitutional requirement for participation in the Medicaid and Medicare programs. But the judge, a Biden appointee, said the companies have not shown that they are legally obligated to sell drugs to Medicare and Medicaid patients.
This is a setback for the pharmaceutical industry’s strategy of getting split decisions in lower courts across the country and ultimately getting the attention of the Supreme Court.
Read more from STAT’s Rachel Cohrs about the details of the ruling.
FDA begins regulating lab-developed tests
From Theranos’ infamous blood tests to misleading prenatal genetic tests, lab-developed tests have historically not been subject to FDA oversight – until now.
The agency released final rules yesterday detailing how it will regulate these tests. The rules are more relaxed than an earlier plan the FDA proposed last year: They give labs four years to comply and they opt not to regulate some tests. Still, the agency estimates that about 12,000 labs will have to submit tests.
The move comes after Congress failed to pass a law that would have allowed the FDA to regulate laboratory tests through a more flexible and cheaper framework. Now that the FDA has taken matters into its own hands, it is expected to face legal challenges in the future.
Read more from STAT’s Lizzy Lawrence about the likely legal battle to follow and what the industry and patients think about the rules.
FDA wants to hear your thoughts on advisory committees
The agency also announced this yesterday a “listening session” will take place on June 13 to hear what the public thinks about the role of advisory committees.
These committees are groups of medical and scientific experts who provide advice to the FDA on regulatory decisions. Their recommendations are nonbinding, but observers closely watch how they vote as a sign of whether the agency might approve or reject a drug or device. However, the FDA has made controversial decisions that run counter to how committees voted, such as granting accelerated approval to Biogen’s Alzheimer’s drug Aduhelm after an advisory committee voted against it.
FDA Commissioner Robert Califf has said he would like to get rid of the voting process. He previously told STAT that “the goal is not to vote on the approval of a product. The goal for me is to get advice from different types of experts.” But Richard Pazdur, the FDA’s chief of oncology, thinks differently and says the votes are useful.
Patients are sour about the pharmaceutical industry
The pharmaceutical industry enjoyed a fairly good reputation during the Covid-19 pandemic, as companies quickly developed treatments and vaccines to use against the virus. But that is changing as patients become increasingly concerned about drug prices and growing shortages.
In an annual survey of more than 2,500 patient groups, 57% said the pharmaceutical industry has an ‘excellent’ or ‘good’ reputation, a decline from previous years. Groups expressed a desire to see drugs become more equitably priced and accessible to patients around the world, and to be more involved in research and development.
Read more from STAT’s Ed Silverman about the detailed results of the survey and which companies have the highest and lowest reputations.
Read more
- What we’re starting to learn about H5N1 in cows and the risk to humans, STAT
- Activist Investor Alex Denner and Bioverativ Shareholder Reach Preliminary Settlement in Insider Trading Case, STAT
- DNA sequencing company PacBio to lay off employees and close its San Diego office Endpoints