Health
Often used alone or with other cancer treatments
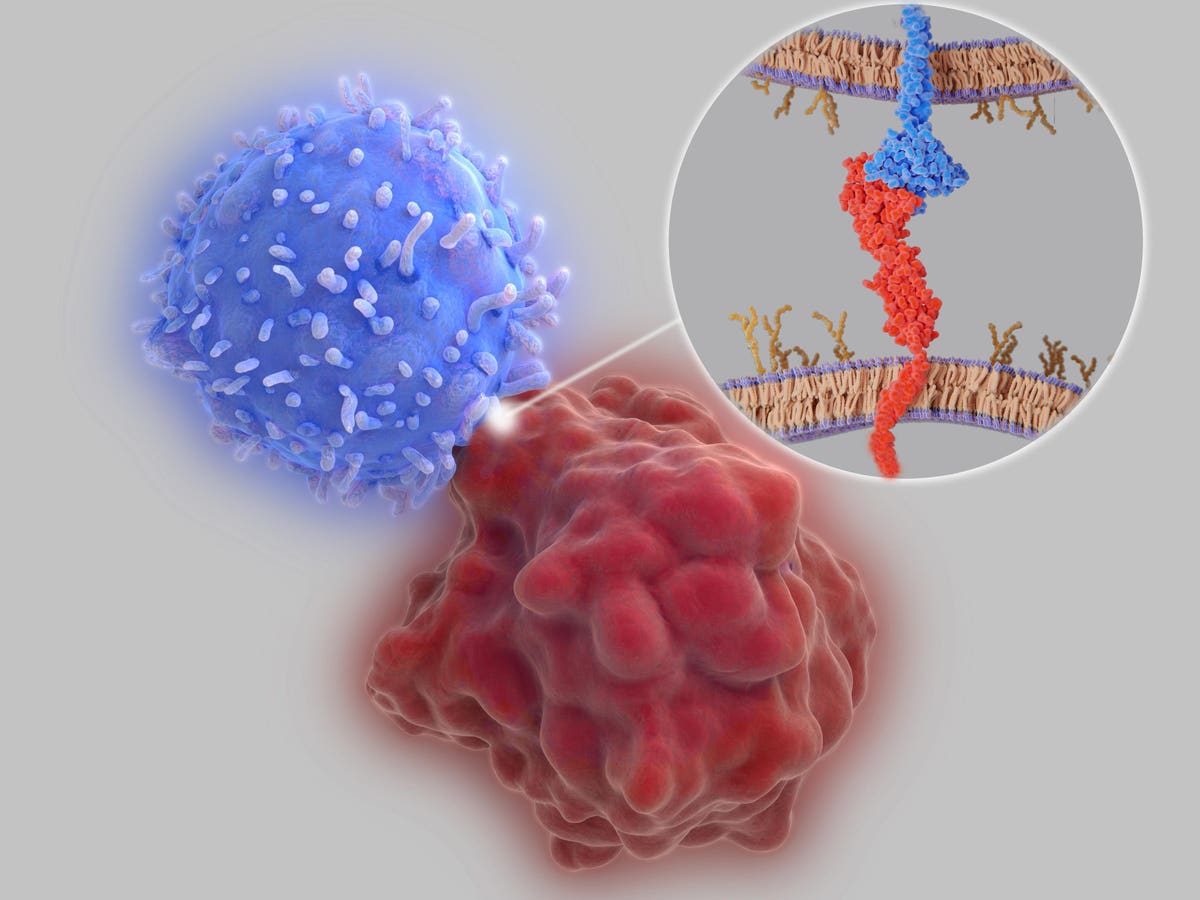
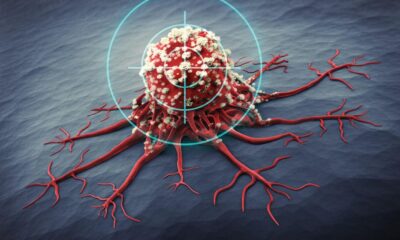
Immune checkpoints are regulators of the immune system. The interaction between PD-L1 (red molecule) … [+]
The 2010s saw the emergence of a new era of cancer care with the development of checkpoint inhibitor therapies. These treatments improved the immune system’s innate ability to kill tumors. Pembrolizumab, by Merck & Co. sold as Keytruda, was one of the first inhibitors to hit the market. A standalone pioneer, similarly functioning checkpoint inhibitors, emerged in the wake of the 2014 approval of pembrolizumab. This article explains how the therapy works, what sets it apart from other inhibitors, and points to possible future developments.
How Pembrolizumab works
Pembrolizumab is a cancer treatment that works by delivering antibodies intravenously into the bloodstream. The antibodies promote anti-tumor responses by binding to immune checkpoints, proteins found on the surface of immune cells.
Under normal circumstances, checkpoint proteins suppress the immune system – both to promote immune tolerance, the process by which the body recognizes and avoids its own healthy cells, and to prevent overstimulated immune responses. Tumors also hijack this system to grow freely. Cancer cells quiet immune cells that would otherwise attack by binding to these checkpoint proteins. Checkpoint inhibitors are valued for releasing these T cells that would normally be suppressed by the tumor, allowing them to launch an attack.
Pembrolizumab encourages T cells to fight by releasing their limitations. The therapy targets a specific checkpoint protein found primarily on T cells, known as PD-1, or programmed death cell protein 1. By blocking access to the PD-1 checkpoint, other cells can no longer use that protein to suppress T cell activity. The T cell can then activate appropriately and take revenge.
Figure 1: PD-1 checkpoint inhibitor mechanism. Pembrolizumab antibodies target PD-1 (programmed cell … [+]
When is Pembrolizumab used?
Pembrolizumab may be a powerful anticancer therapy. It is normally used for advanced cancers that have spread, relapsed after previous treatments, or cannot be removed with surgery alone. Patients receive an infusion every 3 to 6 weeks for up to two years.
How the immunotherapy is administered depends on the disease. For some cancers, doctors may offer pembrolizumab as a first-line treatment. This is the case for qualifying patients with non-small cell lung cancer, throat cancer, stomach cancer or kidney cancer. It can also be given before or after surgery to prevent cancer recurrence. Other times, patients need to try other treatment alternatives before choosing pembrolizumab.
Adding to the complexity, pembrolizumab can be administered alone or alongside other cancer treatments. It is often combined with chemotherapy, radiation and anti-cancer therapies; however, it is not often combined with other checkpoint inhibitors.
The treatment was initially approved for treating humans advanced melanoma. Today, it induces antitumor responses in approximately 18 different types of cancer, including certain blood cancers and several solid tumors. This compatibility can likely be attributed to its broad mechanism of action, as many tumors exploit PD-1 checkpoints to suppress the immune system.
However, keep in mind that the effectiveness of the drug is not uniform. Research shows that pembrolizumab shows improved efficacy in people with the following cancers:
- Melanoma
- Non-small cell lung cancer
- Squamous cell carcinoma of the head and neck
- Classical Hodgkin’s lymphoma
- Solid tumors with certain gene mutations
This inhibitor may also clinically benefit other patients, albeit to a lesser extent; kidney, bladder, liver, colorectal and breast cancer are in this list.
The first approved PD-1 Checkpoint inhibitor
From the superior checkpoint inhibitors On the market, five of these drugs target PD-1 checkpoint proteins, including pembrolizumab. How is pembrolizumab different from these other inhibitors?
Pembrolizumab was the first anti-PD-1 therapy administered federally approval in 2014. In fact, the very first inhibitor approved was ipilimumab, which, as previously described, acts on another checkpoint molecule called CTLA-4. All other PD-1 targeting inhibitors arrived after pembrolizumab.
Another difference is the way the immunotherapy is made. Pembrolizumab relies on humanized antibodies. By comparison, its PD-1 competitors use fully human antibodies.
Finally, pembrolizumab is indicated for the treatment of at least 18 types of cancer. It is used in more combinations against more tumors than ipilimumab or any other federally approved checkpoint inhibitor.
Diagnostic test
A diagnostic test has been developed to help determine which patients would respond best to pembrolizumab. To start the test, a tissue sample is removed from the tumor. Pathologists then analyze the sample in the laboratory to see if the tumor cells express a receptor called PD-L1 (programmed death ligand 1) and at what concentration. Tumors that highly express this protein interact more readily with PD-1 checkpoints on T cells and should therefore be more responsive to pembrolizumab’s anti-PD-1 mechanism. The test is available for patients with advanced squamous cell carcinoma of the head and neck (HNSCC), or for people with certain breast, cervical, stomach or non–small cell lung cancers.
Possible side effects
Due to their mechanism of action, all checkpoint inhibitors engage the immune system and can cause adverse reactions immune-related reactions as a result. In the case of pembrolizumab, PD-1 interactions are crucial for maintaining immune tolerance; releasing this activity can therefore cause several autoimmune diseases. These diseases include colitis, which resembles inflammatory bowel disease, liver toxicity, adrenal and thyroid disorders, and even type 1 diabetes in a minority of patients. Mild to life-threatening inflammation can develop in the kidneys, lungs and other organs.
Patients often experience rashes, fatigue, nausea, diarrhea, loss of appetite, shortness of breath, headaches, musculoskeletal pain, and more.
Combination treatments carry their own additional risks. For example, administering pembrolizumab together with axitinib, a targeted cancer drug, can increase liver toxicity.
Blood tests are required before and after each infusion to closely monitor possible side effects. Treatment may be stopped altogether if side effects are too severe.
Future directions
Pembrolizumab has evolved significantly since its introduction over a decade ago – not in formulation, but in application and therapeutic potential. Ongoing research continues to refine the therapy’s efficacy and safety profile. Future research aims to answer crucial questions, such as whether pembrolizumab should be given before and after surgery, how the drug might be combined with other cancer treatment modalities, and how adjustments in dosing or scheduling may improve patient survival and affect overall response rates.
Takeaways
Pembrolizumab is a transformative anticancer drug with broad applicability and a meaningful impact on patient survival. The treatment shows remarkable response rates for several advanced cancers, many of which resist other cancer treatments. Looking ahead, the research will continue to clarify pembrolizumab’s potential synergies with other treatments and its optimal use in different cancer settings. Future articles will discuss checkpoint inhibitors that use a similar mechanism of action.